IEEE 11073-20701 became ISO standard
Mission accomplished for the IEEE 11073 SDC Core Standards. As the architecture and binding standard is now an official ISO standard, ISO/IEEE 11073-20701:2020, the whole group of SDC Core Standards is approved and published by ISO.
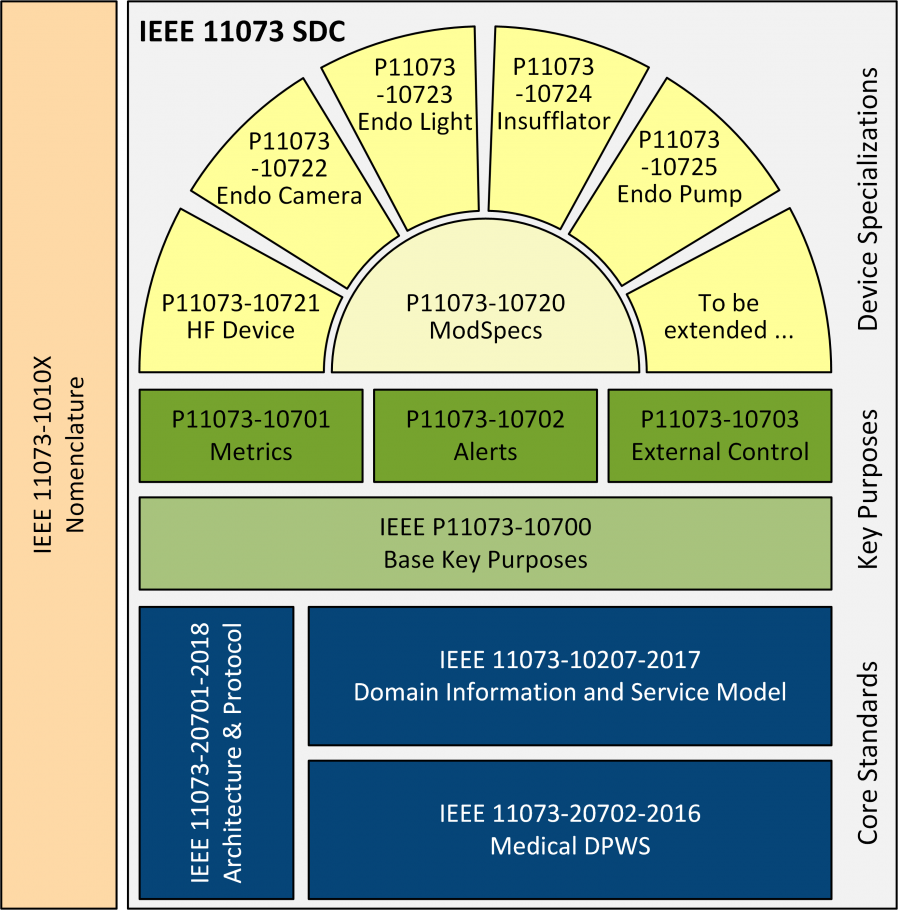
However, this is just the first step. OR.NET association and its members are working hard on further standards: The SDC Core Standards provide the exchange protocol. The so-called Participant Key Interoperability Purpose (PKIP) standards will define process requirements for the different roles medical devices can play in an interoperable device ensemble, like information provider/consumer, alert provider/consumer, or external control provider/consumer.
The most specific level is formed by the Device Specializations (DevSpecs). DevSpecs will device the requirements and device models for particular classes of devices, like high frequency surgical equipment, and endoscopic surgical devices being defined in the PoCSpec project.
We are looking forward to a manufacturer-independent interoperable future of medical devices: Run SDC!


0 Comments
Leave a comment