(Deutsch) SDC-Deep Dive in den Lebenszyklus vernetzter Medizinprodukte (Webinar)
Sorry, this entry is only available in German.
Read moreSDC on Medica 2023
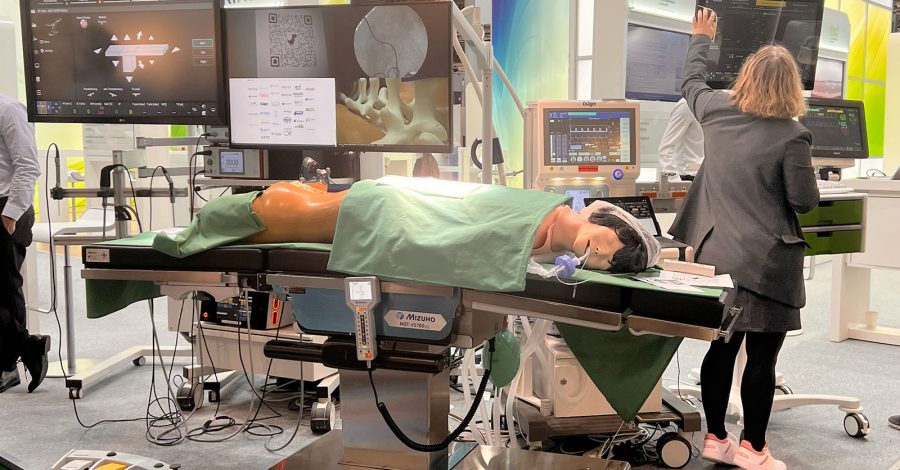
At Medica in Düsseldorf, we will be exhibiting the SDC OR demonstrator with open networking of devices (surgery and anesthesia). You can try everything out for yourself and see that SDC is no longer a vision – but a...
Read more(Deutsch) OR.NET auf der Medica 2023
Sorry, this entry is only available in German.
Read more(Deutsch) SDC Whitepaper of one of our members
One of our members published a whitepaper regarding the connection of legacy medical devices to an SDC network. To get more information you have to register on the website of auriga inc. Link to the whiterpaper
Read moreOR.NET e.V. is now part of the HL7 community
OR.NET e.V. is proud to announce that it has recently joined the HL7 community. Our aim is to work together on Medical Device Interoperability, from the Device Interface to enterprise Health IT systems, using standards such as IEEE 11073...
Read moreIEEE 11073-20701 became ISO standard
IEEE 11073-20701 became ISO standard Mission accomplished for the IEEE 11073 SDC Core Standards. As the architecture and binding standard is now an official ISO standard, ISO/IEEE 11073-20701:2020, the whole group of SDC Core Standards is approved and published...
Read moreApproval strategies for open medical device networking
In a full-day workshop at the notified body DQS Med, members of OR.NET e.V. together with Mr. Bothe from DQS Med discussed the current approval strategy for open networking of medical devices. The distribution of functions creates new opportunities...
Read more